PM [D02] de Broglie deriving the Equation
The de Broglie equation is one of the equations that is commonly used to define the wave properties of matter. It basically describes the wave nature of the electron. Electromagnetic radiation exhibits the dual nature of a particle (having a momentum) and wave (expressed in frequency and wavelength).

Structure Of Atom Concepts
Compton's formula established that an electromagnetic wave can behave like a particle of light when interacting with matter. In 1924, Louis de Broglie proposed a new speculative hypothesis that electrons and other particles of matter can behave like waves. Today, this idea is known as de Broglie's hypothesis of matter waves.In 1926, De Broglie's hypothesis, together with Bohr's early.

PPT Chapter 5 PowerPoint Presentation, free download ID3201239
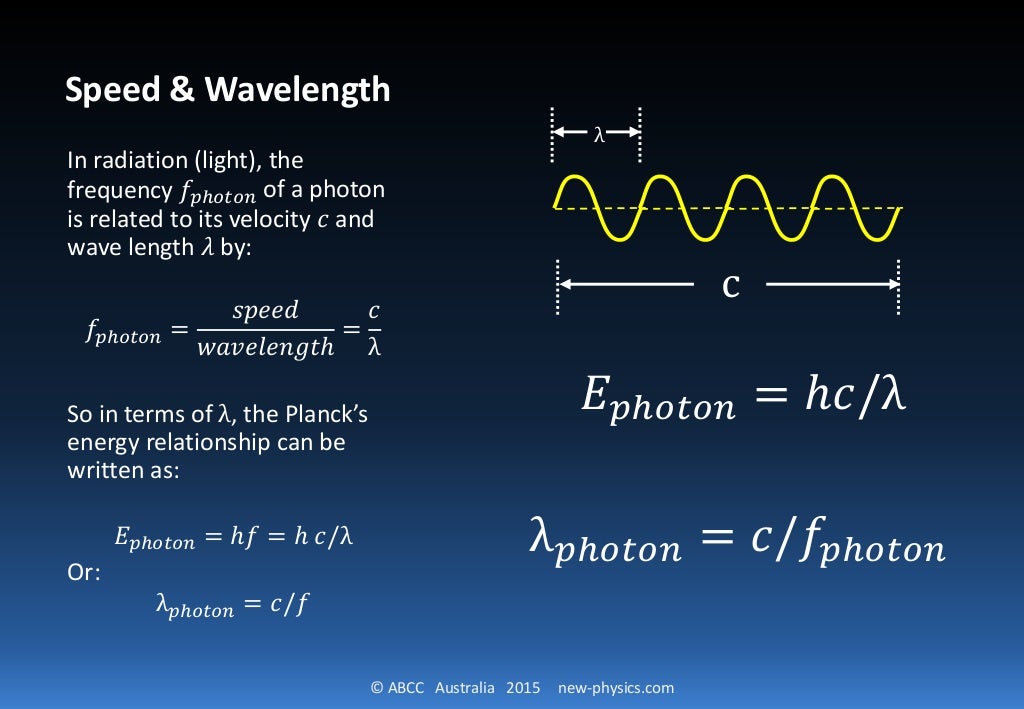
The De Broglie wavelength of a particle is derived by using the formulas for its energy. Consider a photon of mass m with energy as E, wavelength as λ and velocity equal to speed of light, c. The energy (E) of a photon is given as, E = hc/λ ⇢ (1) Also we know that, E = mc 2 ⇢ (2) Equating (1) and (2) we get, hc/λ = mc 2. h/λ = mc.

Двојна природа микрочестица Физика и Оптика
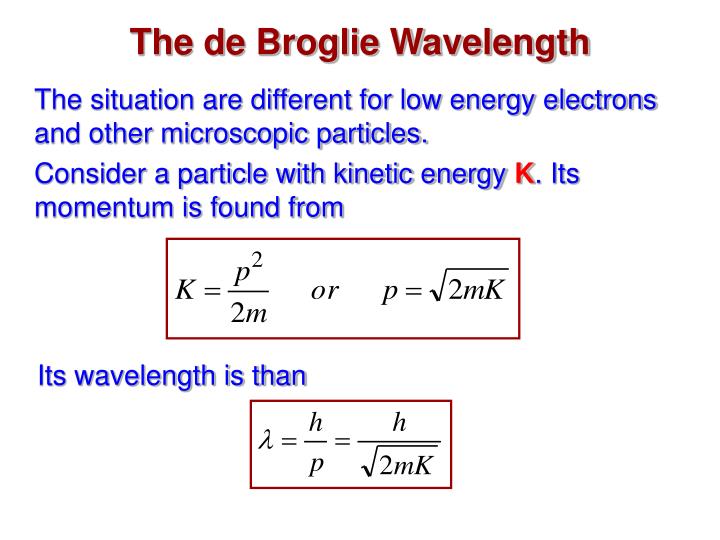
Solution: Reasoning: The de Broglie wavelength of an object is defined as λ = h/p. Details of the calculation: λ = h/p, E = p 2 / (2m), p = √ (2mE), λ = h/√ (2mE). The energy of the electron is 25000 eV * 1.6*10 -19 J/eV = 4*10 -15 J. λ = (6.626*10 -34 Js)/√ (2*9.1*10 -31 kg*4*10 -15 J) = 7.8*10 -12 m.

The De Broglie Wavelength Equation YouTube
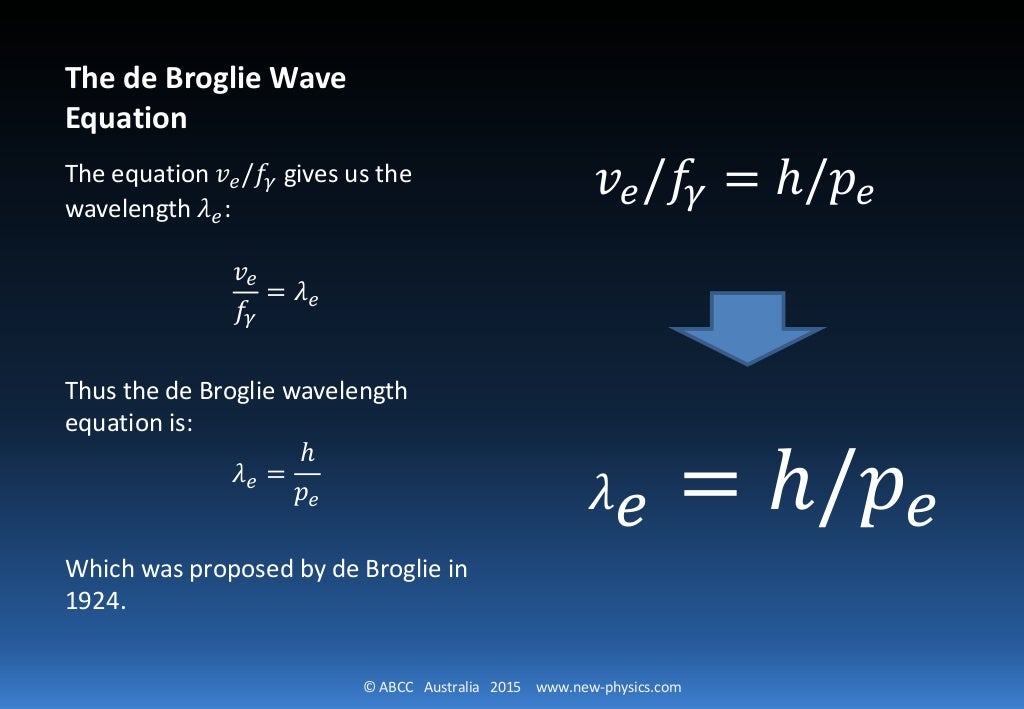
De Broglie was able to mathematically determine what the wavelength of an electron should be by connecting Albert Einstein's mass-energy equivalency equation (E = mc 2) with Planck's equation (E = hf), the wave speed equation (v = λf ) and momentum in a series of substitutions.

Question Video Relating Momentum to the de Broglie Wavelength Nagwa
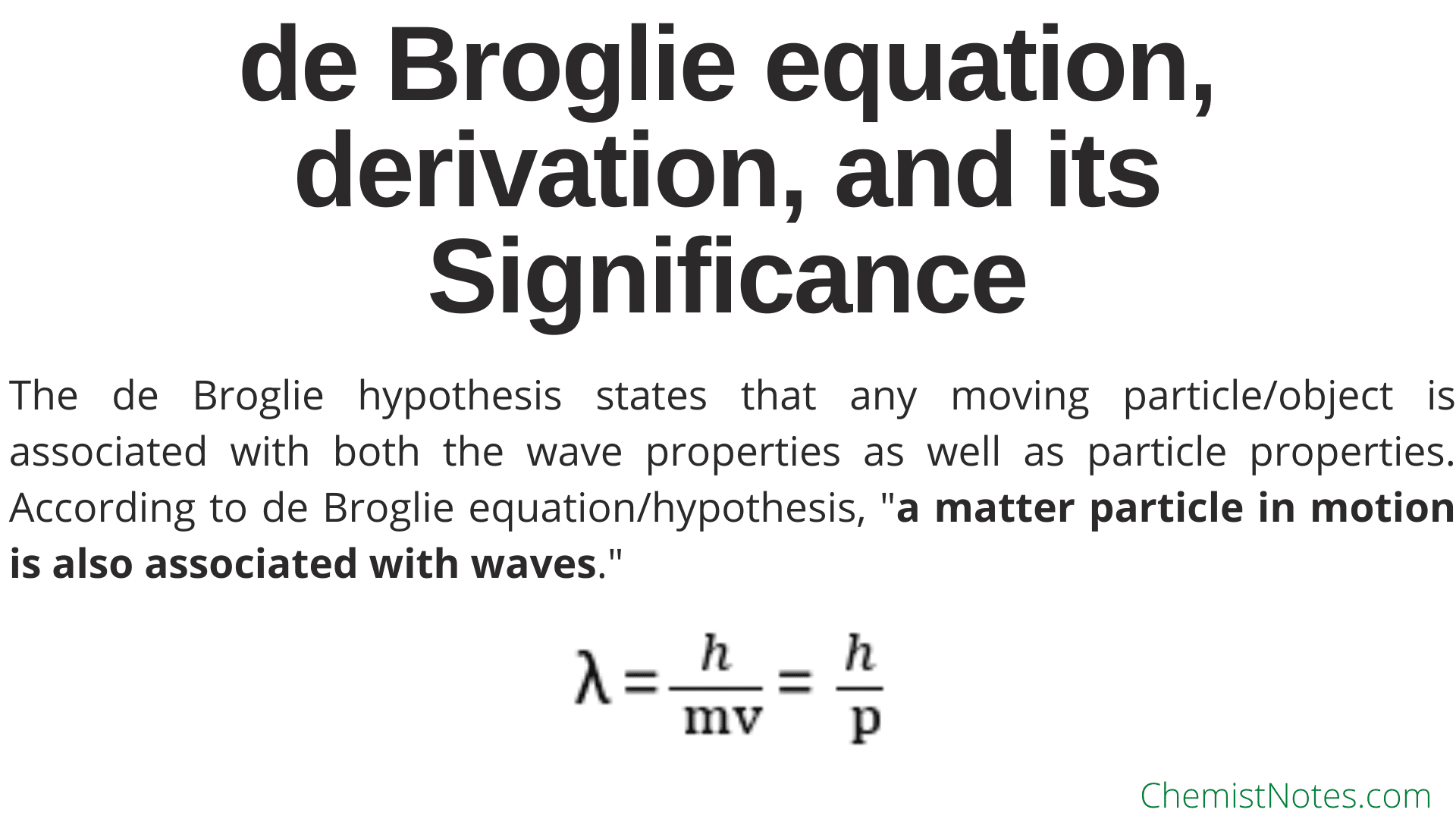
De Broglie Wavelength Matter waves are the central part of the theory of quantum mechanics. All matter can exhibit wave-like behaviour. The concept that matter behaves like a wave this concept was proposed by a French physicist named Louis de Broglie in the year 1924. It is also known as the de Broglie hypothesis.

Question Video Identifying the de Broglie Relationship Nagwa
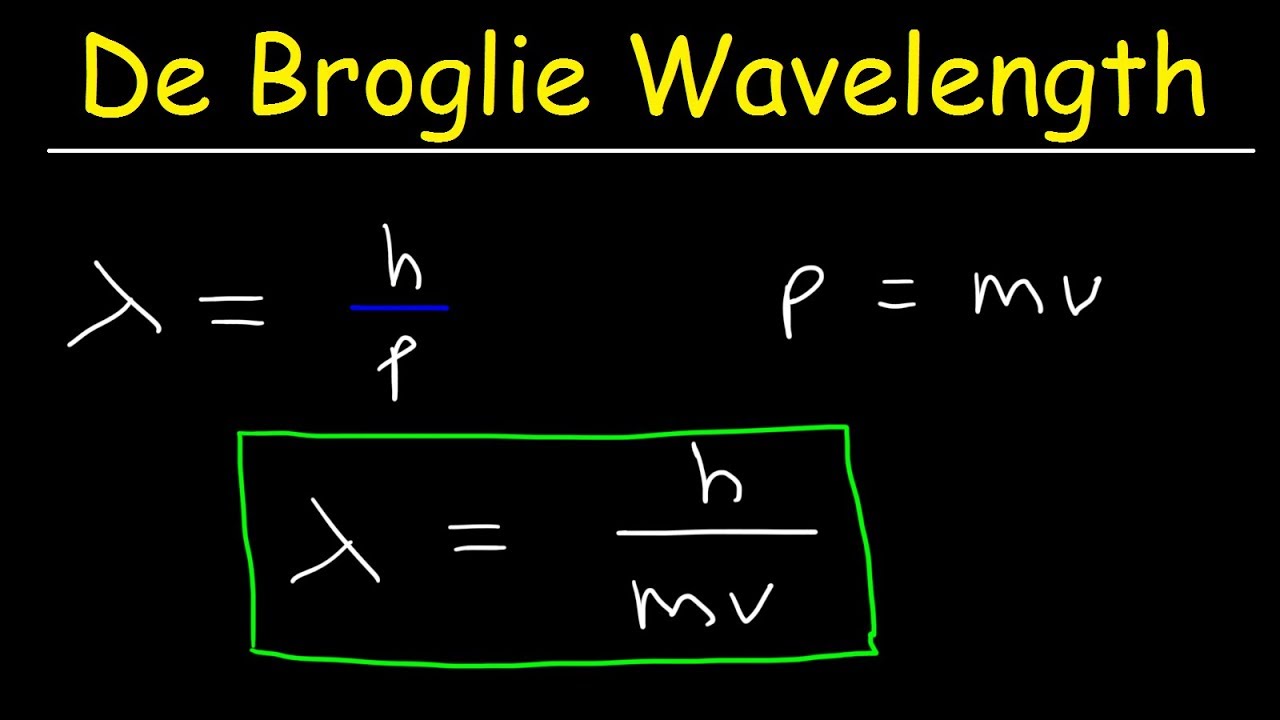
To determine the de Broglie wavelength of a particle given its mass and velocity, you need to: Multiply the velocity by mass. Their product is the particle's momentum. Divide Planck's constant by the momentum found in Step 1. The result you've got is exactly the de Broglie wavelength of your particle. Congrats!
PPT Chapter 5 PowerPoint Presentation ID6630200
Through the equation λ λ, de Broglie substituted v/λ v / λ for ν ν and arrived at the final expression that relates wavelength and particle with speed. mv2 = hv λ (5) (5) m v 2 = h v λ. Hence. λ = hv mv2 = h mv (6) (6) λ = h v m v 2 = h m v. A majority of Wave-Particle Duality problems are simple plug and chug via Equation 6 6 with.

De Broglie Wavelength Formula Formula, Derivation, Applications
This chemistry video tutorial explains how to calculate the de broglie wavelength of large objects and small particles such as electrons. It contains plenty.
What is the significance of de Broglie's equation? Quora
The de Broglie wavelength of the photon can be computed using the formula: λ = h p = 6.63×10−34 1.50×10−27 = 4.42 ×10−7 = 442 ×10−9 = 442 Nano meter. Therefore, the de Broglie wavelength of the photon will be 442 nm. This wavelength will be in the blue-violet part of the visible light spectrum.

De Broglie Wavelength Formula Equation, Concept, Solved Examples
De Broglie hypothesis Propagation of de Broglie waves in one dimension - real part of the complex amplitude is blue, imaginary part is green. The probability (shown as the color opacity) of finding the particle at a given point x is spread out like a waveform; there is no definite position of the particle.
De Broglie wave equation Derivation by SK
Derivation de Broglie derived the above relationship as follows: 1) E = hν for a photon and λν = c for an electromagnetic wave. 2) E = mc 2, means λ = h/mc, which is equivalent to λ = h/p. Note: m is the relativistic mass and not the rest mass since the rest mass of a photon is zero.

de Broglie Equation — Overview & Calculations Expii
De Broglie Wavelength Formula is a formula that defines the nature of a wave to that of a particle. Many experiments show that light can behave both as a wave and as a particle. The particles of light are known as photons.

Question Video Calculating the de Broglie Wavelength of a Particle Nagwa
In 1924, French scientist Louis de Broglie derived an equation, known as the De Broglie Wavelength Formula, that described the wave nature of any particle. Thus, establishing the wave-particle duality for the matter. Microscopic particle-like electrons also proved to possess this dual nature property.
de Broglie equation, derivation, and its Significance Chemistry Notes
de Broglie Wave Equation. Planck's investigation of the emission spectra of hot objects and the subsequent studies into the photoelectric effect had proven that light was capable of behaving both as a wave and as a particle. It seemed reasonable to wonder if electrons could also have a dual wave-particle nature. In 1924, French scientist Louis.
De Broglie theory (Duality) Overall Science
De Broglie's hypothesis is an independent postulate about the structure of nature. In this respect, its status is the same as that of Newton's laws or the laws of thermodynamics. Nonetheless, we can construct a line of thought that is probably similar to de Broglie's, recognizing that these are heuristic arguments and not logical.